To convert grams into moles, it is necessary to be aware of the element’s molar mass you want to determine. The Molar mass is a primary number you can find through the periodic table searching for the element’s symbol. The atomic mass is then rounded to the nearest number of whole numbers. To determine a compound’s molar mass, you need to divide all weights with the abundance of every individual atom.
How many moles are in 22 grams of argon?
It is 39.948. We presume that you’re switching from kilograms Argon or mole.
The molecular formula of Argon has the formula Ar.
The SI base unit for the quantity of the substance is called the mole.
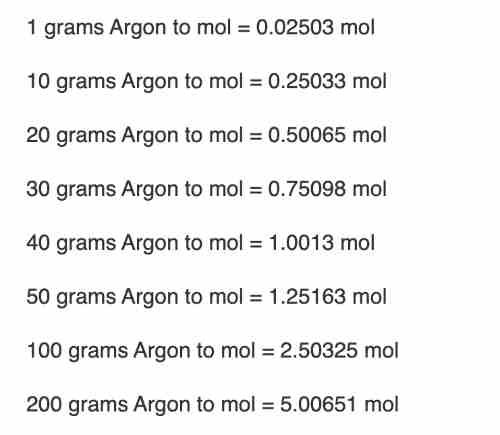
One gram of Argon is equivalent to the equivalent of 0.025032542304996 moles.